Legislation: European Food Law
European Food Law is a complex and broad topic to work with. The General Food Law Regulation ensures a high level of protection of human life and consumers’ interests in relation to food while ensuring the effective functioning of the internal market.
To quickly assess whether a certain food product or ingredient is allowed to be imported into the EU market is often a question that has a larger list of sub-questions to be answered. The good news is that the European Food Law Regulation is strict, but it delivers a level playing field for all parties in a large region. Also, the regulations are applicable in twenty-seven member states, and only minor details may be existent in an individual member state. At QAssurance, we are delivering an assessment tool that allows for a quick scan of your portfolio of food products or ingredients. Taking into account that details in this field define the final verdict, the tool will give an 80/20* insight into your portfolio. With that insight, you can decide to spend more time on details, depending on your strategic needs.

* For many products or ingredients in your portfolio, you will get a clear answer about the opportunity to use those in the EU market. For a smaller set of products, you will be guided towards the details that have to be confirmed before being able to get clearance.
Details:
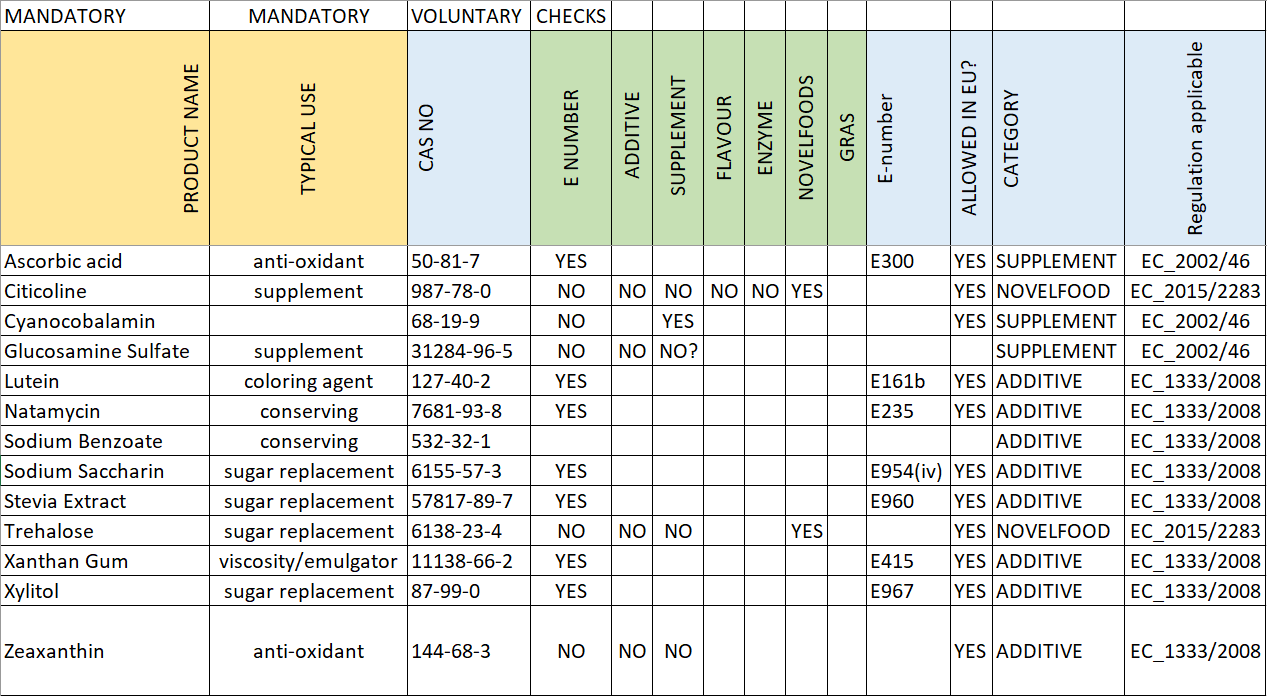
For optimal output of the assessment tool, a few details on the products/ingredients will have to be supplied. QAssurance uses a straightforward Excel template for that.
- Product Name: Describe the name as specific as possible.
- Typical use: Colour, preservative, antioxidant, sweetener, emulsifier, stabiliser, thickener, etc.
- CAS no.: CAS no. is helpful to define the specific material
Checks are done on each product/ingredient:
- E NUMBER
- ADDITIVE
- SUPPLEMENT
- FLAVOUR
- ENZYME
- NOVEL FOODS
For each product/ingrediënt, the final result will be given as well as the typical use that is allowed.
Process description:
The steps below are a draft and need optimizing in time. When using this process with a list of products/ingredients, make sure to be aware of additional tests to be added to the steps or improve the steps as they are set out.
Use the Excel template and run the steps below.
STEPS:
| STEP | WHAT | |
| Check for completeness. Only products for which all details are entered can be assessed. Before checks, make sure all links below are still valid. | ||
| Run 6 checks and If check = true, then USE = YES, and insert valid check as ‘allowed as’ | ||
| 1 | E numbers check against the list https://en.wikipedia.org/wiki/E_number#Full_list and when found, fill in the E number in column I (TBD). | |
| 2 | Additive, check against https://food.ec.europa.eu/safety/food-improvement-agents/additives/database_en | |
| 3 | Supplement check against 2002/046 https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0046&from=EN | |
| 3 | Supplement also check at https://ec.europa.eu/food/safety/labelling_nutrition/supplements_en. This has 6 directives that need to be checked. | |
| 4 | Flavour, check against https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1334&from=EN ATTENTION; Annex ii part A is a list of substances NOT to be added to food. | |
| 5 | Enzyme, check against https://food.ec.europa.eu/safety/food-improvement-agents/enzymes_en . If YES, the state the allowed enzyme name in column M. | |
| 6 | NovelFoods (make sure that expand all 3* is on and retype search text every time) https://food.ec.europa.eu/safety/novel-food/authorisations_en if YES as novel food, state dossier number in column TBD. | |
| 6 | 2e check https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R2470&from=NL | |
| IF USE = NO, Google product for synonyms and re-run steps and check if the product is GRAS: Generally Recognized AS SAFE | ||
| Google product and find news or other typical use, sometimes products are under debate and this should be mentioned in excel. |
Report the results through the completed Excel file and add additional points of attention found in the process.
Add a disclaimer section in the report with the elements below:
ASSUMPTIONS/ DISCLAIMER
- Assumption 1, products/ingredients do not originate from animal sources. In case the product originates from an animal source, documentation that it is from an EG registered company is always required.
- Assumption 2, products/ingredients are GMO-free. If not GMO-free, further checks against regulations for this type of product are required as a next step.
- Most additives are only permitted to be used in certain foods and are subject to specific quantitative limits, so it is essential to use them in conjunction with the appropriate legislation.
- The assessment is only valid for the type of use given, as stated in column K (ALLOWED AS).
- For this assessment, the client’s product/ingredient names and descriptions are evaluated as supplied. The safety of the actual products/ingredients will have to be assessed.
Related articles to Food materials for European market clearance
Many customers and visitors to this page 'Food materials for European market clearance' also viewed the articles and manuals listed below:
