Introduction
The European Union is taking decisive steps to enhance the safety of plastic food contact materials (FCMs). Driven by a commitment to protecting consumer health and minimizing potential risks associated with chemical migration, the EU has implemented significant updates to its regulations. Regulation (EU) 2025/351, which entered into force on March 16, 2025, introduces stricter requirements for the composition, manufacturing, and labeling of plastic FCMs.
These changes impact businesses throughout the food supply chain, from material suppliers and packaging manufacturers to food producers and retailers. This overview will detail the key amendments introduced by this regulation, highlighting their practical implications and placing them within the broader context of EU efforts to ensure the safety of all materials that come into contact with our food.
The Existing Framework: Key Food Contact Materials Regulations
Before diving into the upcoming changes, it’s crucial to understand the foundation upon which they’re built. The EU has a comprehensive and evolving framework of regulations governing FCMs.
This existing framework isn’t static; it’s a layered system, with general principles and specific rules for different materials and substances:
- Regulation (EC) No 1935/2004 – Overarching framework regulation for all food contact materials. Establishes the general principle that FCMs must not release their constituents into food at levels harmful to human health or unacceptably change the food’s composition.
- Regulation (EU) No 10/2011 – Specific regulation for plastic FCMs. It sets out detailed rules on composition, migration limits, and compliance testing.
- Regulation (EU) 2022/1616 – Focuses on recycled plastic FCMs, establishing requirements for authorization of recycling processes and ensuring the safety of recycled materials.
- Regulation (EC) No 2023/2006 – Sets out Good Manufacturing Practice (GMP) requirements for all FCMs, including aspects like quality control and documentation.
The Core Change: Regulation (EU) 2025/351
Commission Regulation (EU) 2025/351, in force since March 16, 2025, significantly transforms the EU’s regulatory landscape for plastic food contact materials. It amends three core pieces of legislation: Regulation (EU) No 10/2011, Regulation (EU) 2022/1616, and Regulation (EC) No 2023/2006.
While a transitional period is in place, understanding the key changes is crucial for all businesses in the food supply chain:
Stricter Purity: Everything used to make plastic food packaging – including recycled materials and natural substances – must be pure. This means fewer unwanted chemicals and stricter limits on potentially harmful substances.
More Transparency: Companies must provide more material details, including unintentional substances like impurities and byproducts. The Declaration of Compliance (DoC) now must include information on these substances if they could potentially affect health.
Clearer Rules for Reprocessed Plastic: Regulation clarifies rules for using “reprocessed plastic” (mechanically processed only).
Biocides: Only biocides (substances that kill microorganisms) approved under specific EU rules can be used in plastic food packaging.
Multi-Layer Materials: Migration rules now apply to the food-contact plastic layer in multi-layer packaging.
Repeated Use: Final food packaging that is meant to be used must include labelling regarding how to prevent or slow down its deterioration.
Good Manufacturing Practices (GMP): Clear rules apply to collecting, pre-processing, and reprocessing plastic manufacturing by-products.
Recycled Content: The Declaration of Compliance must now state whether the plastic contains recycled material.

Regulation (EU) 2025/351 is in force as of March 16, 2025. However, a transitional period is established on plastic materials and articles that complied with the previous regulations to be place.
Specific Substance Food Contact Materials Regulations: The Case of BPA
Complementing the general framework governing food contact materials, the European Union employs specific, targeted regulations to manage the risks associated with particular chemical substances.
These measures are implemented when scientific evidence indicates a potential health concern, allowing for stricter controls beyond the general rules for materials like plastics.
- Bisphenol A (BPA) – Responding to updated scientific assessments from the European Food Safety Authority (EFSA) regarding potential risks, particularly to the immune system, the EU implemented Commission Regulation (EU) 2024/3190.
- Epoxy Derivatives: Restrictions on certain epoxy derivatives (like BADGE, BFDGE, and NOGE) often found in coatings and adhesives (Regulation (EC) No 1895/2005).
- N-Nitrosamines: Limits on the release of N-nitrosamines and N-nitrosatable substances from elastomer or rubber teats and soothers (Directive 93/11/EEC).
- Vinyl Chloride Monomer: Strict limits on the presence of vinyl chloride monomer in PVC materials and articles (Directive 78/142/EEC).
What Businesses Need to Do :
Proactive measures are essential for all businesses involved with plastic food contact materials, including those exporting products to the EU market. To ensure continued compliance and market access, consider the following key actions:
- Monitor Regulatory Developments: The proposed Regulation (EU) 2025/351 is not yet law. Businesses, including those exporting to the EU, should closely monitor its progress.
- Assess Current Compliance: Review all plastic FCMs used to ensure they comply with existing regulations, including Regulation (EU) No 10/2011, Regulation (EU) 2022/1616, and the BPA ban (Regulation (EU) 2024/3190), as well as other relevant substance-specific regulations (e.g., 1895/2005/EC, 93/11/EEC, 78/142/EEC).
- Prepare for Stricter Purity Requirements: Begin assessing all substances used, including those from recycled or natural sources, in preparation for the “high degree of purity” requirements. This is particularly important for exporters to the EU, as their materials will need to meet these standards.
- Review Documentation and Supply Chains: Ensure Declarations of Compliance are accurate and complete. Exporters to the EU must be prepared to provide detailed information on NIAS, as required. Close collaboration with suppliers is essential.
- Recycled Plastic Sourcing (for Importers): EU businesses importing food products packaged in recycled plastic must ensure the recycling facility is registered with the EU.
- Recycled Plastic Sourcing (for Exporters): Non-EU businesses exporting food products packaged in recycled plastic must ensure their recycling facilities are registered with the EU, as per the proposed amendments. This is a critical requirement for market access.
- Consult Official Sources: Regularly refer to reliable sources to keep up with updates.
Stay Compliant with Food Contact Materials Regulation with Ease
Navigating the ever-changing landscape of food contact material regulations can be overwhelming. That’s where iMIS Food comes in.
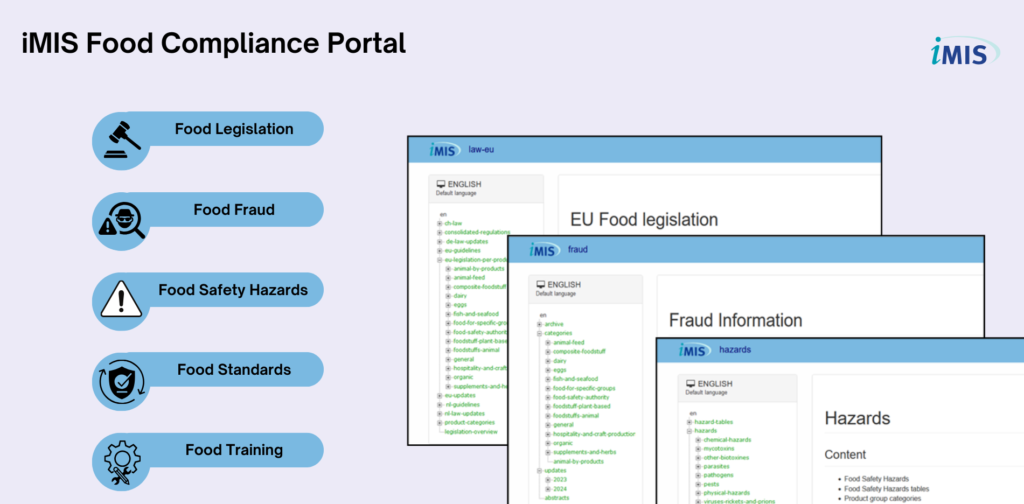
iMIS Food is a comprehensive, user-friendly online platform designed to keep your business effortlessly compliant with the latest EU and global food regulations.
Now online: iMIS Food Compliance
iMIS Food is more than just a database; it’s your proactive compliance partner. It provides:
- Instant Access: Get the latest information on Food Legislation, Food Fraud, Food Safety Hazards, and Food Standards, all in one place.
- Expert-Led Training: Enhance your team’s knowledge with comprehensive Food Training resources.
- Reduced Risk: Minimize the risk of non-compliance and costly penalties.
- Time Savings: Stop wasting time searching – find what you need quickly and easily.
- Continuous Updates: Stay informed with regular updates on evolving regulations.
Ready to streamline your compliance process? Contact: info@qassurance.com
Conclusion
The EU is actively strengthening its regulations on plastic food contact materials. The proposed changes, particularly those outlined in the anticipated Regulation (EU) 2025/351, will require significant adjustments from businesses.
By staying informed, proactively assessing their materials and processes, and engaging with their supply chains, companies can navigate these changes and ensure the continued safety and compliance of their products.
Stay Connected: Join our Newsletter!
Ready to learn more? Subscribe to our newsletter for the latest updates, tips, and exclusive content delivered straight to your inbox!
Sources
- Food Safety (2024, 19 December) – Commission adopts ban of Bisphenol A in food contact materials.
- Directive (EU) 2019/904 of the European Parliament
- European Food Safety Authority. (2024, 19 December) – Food contact material applications: regulations and guidance
- AGRINFO Platform. (2024, 18 maart) – Plastic (including recycled) food contact materials
Related articles to Plastic Food Contact Materials: New EU Rules and BPA Ban
Many customers and visitors to this page 'Plastic Food Contact Materials: New EU Rules and BPA Ban' also viewed the articles and manuals listed below:
